

This meant that they could not be simply charged atoms (ions), or any then known particle. Thomson was ready with his big announcement the cathode rays consisted of negatively charged particles (which he called "corpuscles") that were only less than 1/1000th of the mass of a hydrogen atom.

Just as had happened with a magnetic field, so with the electric field, the rays were bent!īy April 30th, 1897 J.J. By connecting these plates to the opposite poles of a battery, an electric field was generated at right angles to the path of the rays.
#Jj thomson cathode ray experiment explanation series
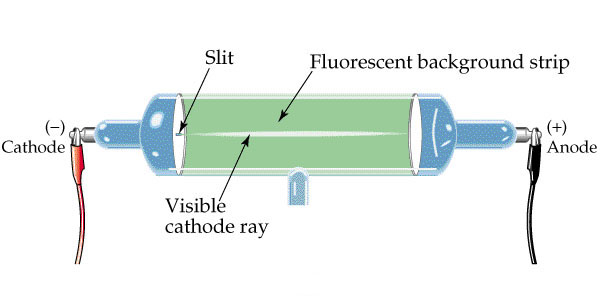
Thomson also carried out a series of experiments using a better designed cathode ray tube that incorporated two small plates, between which the rays had to travel. So it was more likely that they were particles than waves. By 1894 Thomson was able to announce that these rays traveled much more slowly than rays of light (he found a value of 1.9 x 10 7 cm/sec. This observation allowed Arthur Schuster (in 1890) to calculate the charge-to-mass ratio of these "particles" from the amount of bending he observed in a cathode ray tube when it was magnetized.Īll these interesting discoveries attracted the attention of the English physicist Joseph John Thomson ("J.J." - to his friends). William Crookes (an English physicist), among several others, including Julius Plucker, showed that bringing a magnet next to the sides of the tube caused the cathode rays to bend in a way that strongly suggested that they were made up of electrically charged particles - not waves. This difference in concept caused a lot of rivalry at the time and, although none of them were ever to know it, they were both right - sort of! German researchers were convinced that the cathode rays traveled in waves, like light, whereas the British researchers thought that the rays were composed of tiny, tiny charged particles. Since Faraday had called the negative pole or plate the cathode, Goldstein called his radiation, cathode rays. On the wall opposite to the negative cathode, the glass glowed a strange, greenish color.īy 1876 Eugen Goldstein was certain that the glow was being caused by a new kind of radiation that started at the negative plate and radiated across the vacuum until it hit the glass.

His superior vacuum pump removed all the air from the tube, and he connected the anode and the cathode to the appropriate ends of a powerful battery.Īt high enough voltages electricity certainly seemed to be able to leap across the vacuum between the oppositely charged plates, but that was not all. His apparatus consisted of a glass tube in which an anode (the positive pole, or plate) was at one end, and the cathode (the negative pole, or plate) was at the other end. Unfortunately his methods of producing an appropriate vacuum were not good enough and he never really succeeded, but a German glass blower - Heinrich Geissler - certainly did. He also got the idea to pass an electrical current (discharge) through a complete vacuum, just to see what happened - if anything. Using this technique, he discovered many things, including new elements, and aroused the interest of his pupil - Michael Faraday (1792 - 1867).įaraday coined many of the terms still used today, including electrolysis, electrolyte, electrodes, anode, anions, cathode and cations. The English chemist Humphrey Davy (1778-1829) had built the world's largest battery (over 250 metallic plates) and pushed the very high currents this battery could generate through all kinds of solutions, compounds and substances in the hope that the high energies involved would pull apart the chemical constituents. In the 1800s electricity was new, exciting and the subject of a lot of study.


 0 kommentar(er)
0 kommentar(er)
